Exciplex study
Abstract
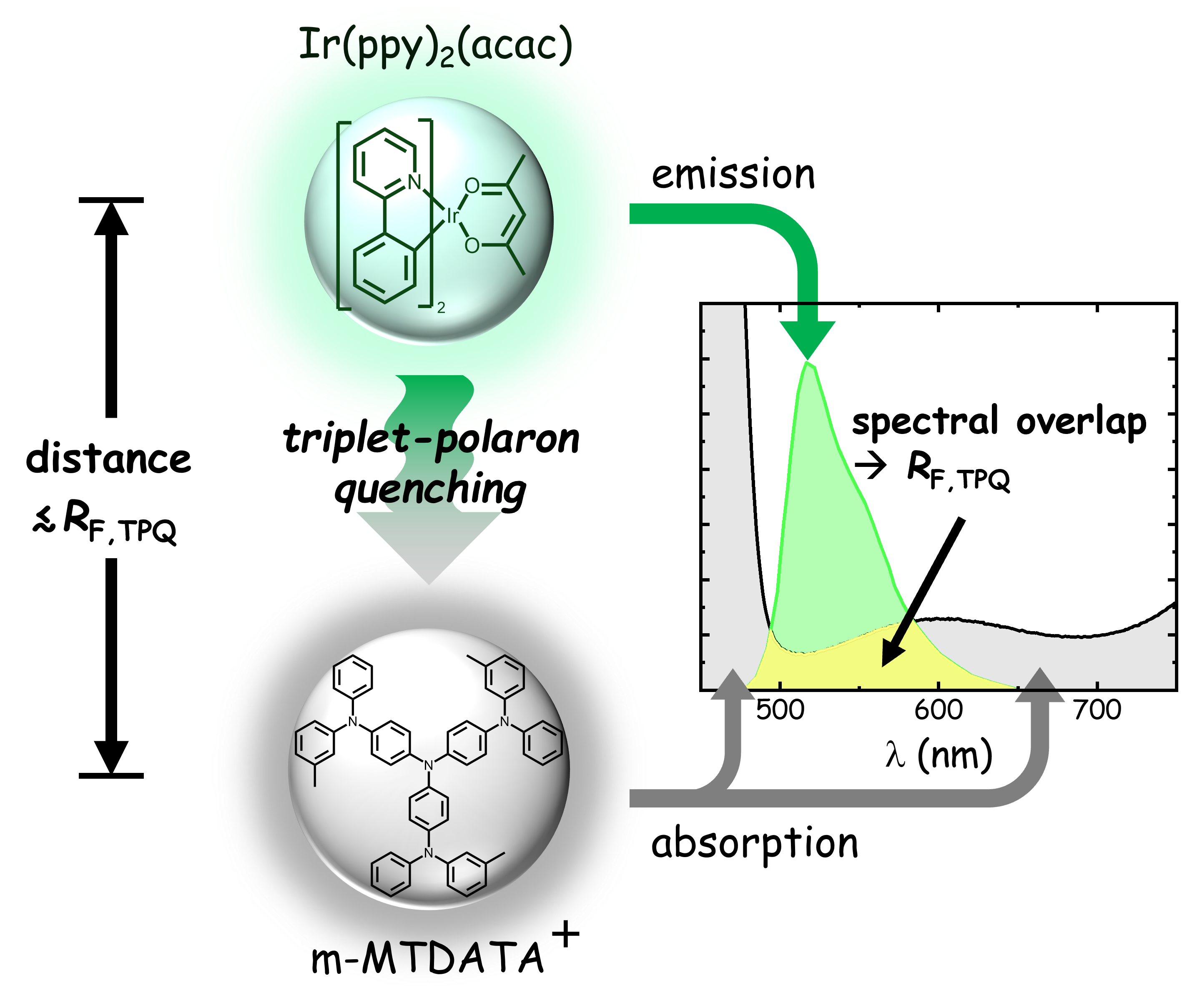
The quantum efficiency of phosphorescent organic light-emitting diodes (OLEDs) shows a decrease with increasing current density and luminance (“roll-off”). A major contribution to the roll-off is triplet-polaron quenching (TPQ), upon which a triplet exciton on an emitter molecule (“donor”) is lost after exciting an electron or hole polaron on another molecule (“acceptor”), followed by non-radiative decay. The microscopic mechanism is not well understood. Within a Förster-type dipole-dipole interaction model, the TPQ rate is determined by the overlap of the donor photoluminescence spectrum and the absorption spectrum of the positively or negatively charged acceptor molecules. In this work, a spectroelectrochemical method is demonstrated for measuring the absorption spectra of charged molecules, so that the interaction rate, expressed in terms of the TPQ Förster radius, can be determined. Typical values of these radii are found to be in the range of 2.5−4 nm. For often used OLED designs, in which emitter molecules (“guests”) are embedded at a small concentration in a matrix material (“host”), this study enables obtaining in a rational manner optimal host-guest combinations. The methodology is also expected to provide an experimental benchmark for emerging open-shell quantum-chemical methods for quantifying TPQ.
This work has been published in Advanced Functional Materials
(Adv. Funct. Mater. 2025, 2425548)